3.2 Entropy and Heat (Thermal Physics) (Schroeder)
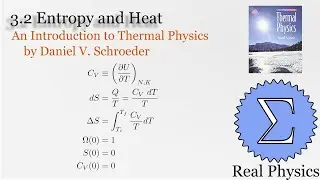
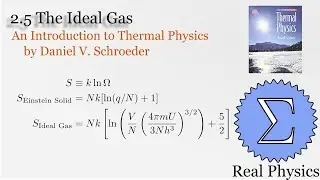
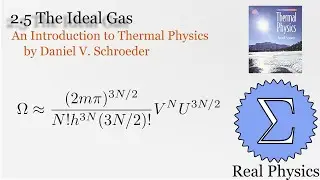
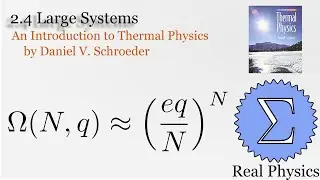
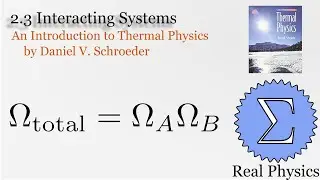
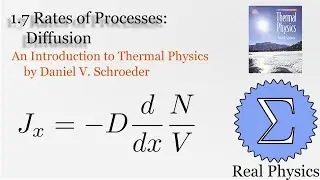
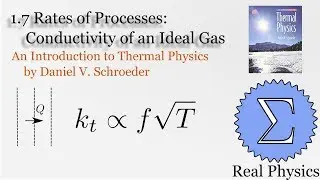
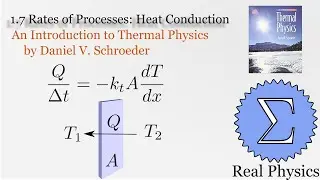
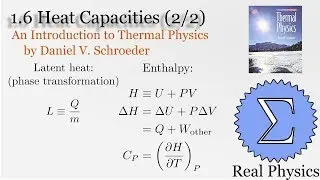
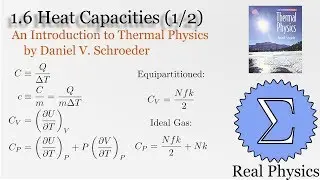
We've seen how temperature and entropy relate, so now let's look at how heat and entropy are related. It all comes down to the heat capacity. The simple formula dS = Q/T is also useful for calculating the entropy when we don't have a convenient model to calculate the multiplicities. We also discuss what happens as T approaches 0, and make some observations about "residual entropy" and how the heat capacity should behave as we approach 0 K.
Playlist: • An Introduction to Thermal Physics by...
Join us on Discord: / discord
You can support this channel by buying the book through this link: https://amzn.to/2Z8xEpX
You can also support the channel by donating to / realphysics . Any amount will greatly help and is very much appreciated.
The textbook I am using is: Schroeder, Daniel V. "An Introduction to Thermal Physics" 1st ed., Addison Wesley Longman, 2005..