3.1 Temperature (Thermal Physics) (Schroeder)
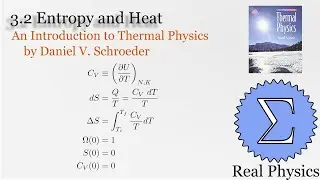
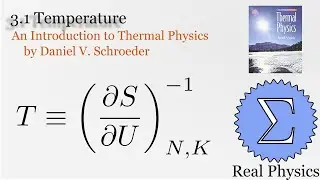
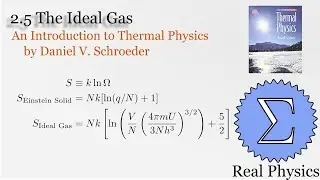
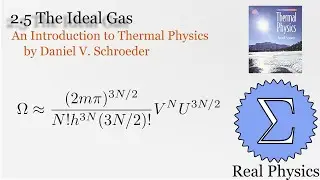
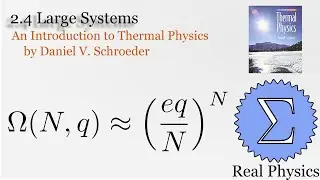
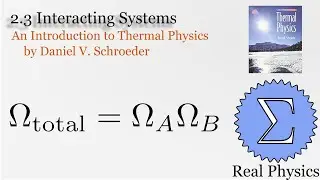
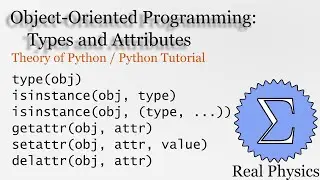
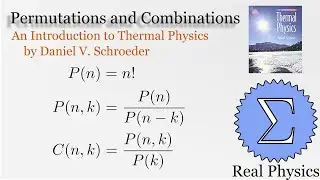
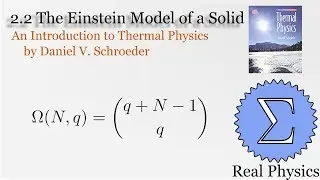
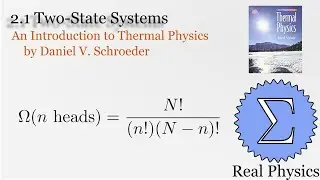
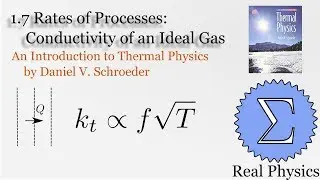
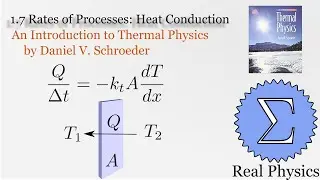
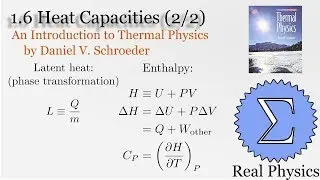
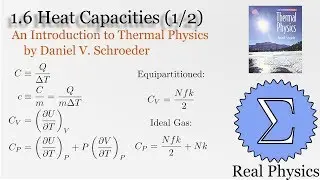
With a solid understanding of entropy, we can now define temperature mathematically. Back in section 1.1, we said that temperature was thing that is the same when two bodies are in thermal equilibrium. We now know that when two bodies come into thermal contact, that the total entropy will be maximized as energy is exchanged between them. So the rate of change in the entropy over energy for each body must be equal. This is the inverse of the temperature. We then calculate the temperature of Einstein Solids and Ideal Gases. I also share Schroeder's "Silly Analogy" to help you understand why this definition of temperature is intuitive and simple.
Playlist: • An Introduction to Thermal Physics by...
Join us on Discord: / discord
You can support this channel by buying the book through this link: https://amzn.to/2Z8xEpX
You can also support the channel by donating to / realphysics . Any amount will greatly help and is very much appreciated.
The textbook I am using is: Schroeder, Daniel V. "An Introduction to Thermal Physics" 1st ed., Addison Wesley Longman, 2005..