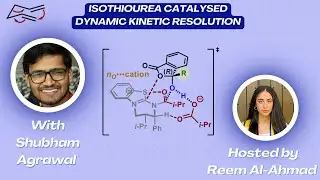
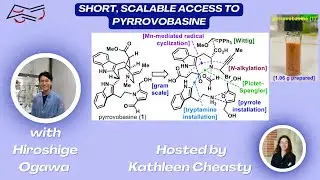
Short, Scalable Access to Pyrrovobasine with Hiroshige Ogawa
In this Research Spotlight hosted by Kathleen Cheasty, Hiroshige Ogawa joins us to highlight the Nakamura group's work on the synthesis of pyrrovobasine.
Key reference:
JACS Au 2023, 3, 3000-3004.
https://doi.org/10.1021/jacsau.3c00595
Additional references (in order of appearance):
For isolation, see Org. Biomol. Chem. 2021, 20, 98-105.
For a review of Maillard-type reactions, see Angew. Chem., Int. Ed. 2014, 53,10316−10329.
Proposed biosynthetic pathway, see Org. Biomol. Chem. 2021, 20, 98-105.
For pioneering work towards key intermediate Org. Lett. 2000, 2, 2057-2059; J. Org. Chem. 2000, 65, 3173−3191.
For bioinspired activation of the tertiary amine moiety, see J. Am. Chem. Soc. 2021, 143, 19966−19974.
For Mn-mediated direct radical cyclization, see J. Org. Chem. 2022, 87, 5690−5702.
For Ir-catalyzed photo epimerization, see Nat. Commun. 2022, 13, 908.
For diflurocarbene mediated ring-opening, see J. Am. Chem. Soc. 2021, 143, 19966–19974.
Eur. J. Org. Chem. 2014, 2014, 1431−1437; Green Chem. 2023, 25, 196−210.