Sulphuric Acid H2SO4 Vs Match stick Vs Sponge | DIY Science Experiment | Unbelievable Experiments
Welcome welcome back to another episode of "DIY" science Acid experiment - Sulfuric Acid (H2SO4) VS Safety Matches vs paper Vs Sponge Vs Flower from The Maverick Lab.
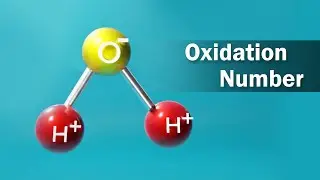
Sulphuric acid (alternative spelling sulphuric acid), also known as vitriol, is a mineral acid composed of the elements sulphur, oxygen and hydrogen, with molecular formula H2SO4. It is a colorless, odorless, and viscous liquid that is soluble in water and is synthesized in reactions that are highly exothermic.
Safety matches use potassium chlorate as an oxidizer, and when it comes into contact with sulfuric acid, they react to become chloric acid. The chloric acid is in turn “extremely reactive and unstable” and proceeds to react with the other chemicals in the match head, burning them and starting a fire.
The sponge contains cellulose, which is made up of carbon, hydrogen and oxygen. Because sulfuric acid wants to react with water so badly, it actually rips the hydrogen and oxygen molecules off the cellulose, causing it to disintegrate.
#ScienceExperiment #DIY #Sponge #SulphuricAcid #experiment #DiyTricks #MatchStick