Isobars - Isotopes | Atomic Structure | CBSE Class 11 Chemistry by Elearnin
Welcome to Elearnin, In this 3d animated videos we will teach you about Isobars and Isotopes from the Class 11 Chemistry - CBSE – NCERT by Elearnin
In this video you will learn about Isobars - Isotopes
0:11 Isobars and Isotopes
0:15 Atomic Number
0:45 Mass Number
2:52 Isotopes
3:37 Isobars
#Isotopes #Isobars #Chemistry #CBSE #3dAnimation #Education #Class11
Atomic Number
The electrons and protons in an atom are equal in the (Atomic Number Z).
For example, in the atoms Hydrogen and Sodium, the number of electrons is 1 and 11 respectively.
Elemental Atomic Number Z = The number of protons in the element’s atom
(or)
The number of electrons in the element’s neutral atom
Mass Number
The protons and neutrons in the nucleus are called Nucleons.
The total number of nucleons is call the Mass Number (A).
Mass Number (A) = Number of protons (Z) + Number of Neutrons (N).
For example, the mass number of F is 19 and the atomic number is 9
The atomic number of Flourine has been written at the the bottom left of it’s symbol ‘F’. It indicates that there are 9 protons in it’s atom. In the same way, its mass number has been written at the top left corner of F.
It shows that Flourine’s nucleons i.e. protons and neutrons number 19 in total.
Therefore, the number of neutrons in Flourine is 19-9 =10.
Because N = A-Z.
The number of neutrons, protons and electrons in an atom are 18, 16 and 16 respectively. Please give the atom its correct symbol.
Practice:
Atomic number = Number of protons = 16
The element is Sulphur ‘S’.
Atom’s Mass number = Number of protons + Number of Neutrons
= 16 + 16 = 32
The number of protons is not equal to the number of electrons. Therefore, the atom is not neutral. It’s an anion (Negatively charged). Equal to the number of excited electrons.
Number of extra electrons = 18-16 = 2
Symbol ‘S’, mass number is 32, atomic number is 16, number of extra electrons is 2.
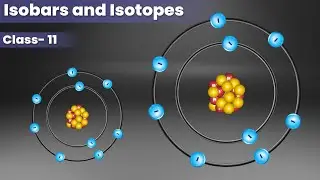
Isotopes
Isotopes are atoms with the same atomic number but different mass number. For example, if you see Hydrogen atoms, 99.985% of hydrogen atoms have only one proton. This isotope is called Protium. Other than this, Hydrogen has two isotopes. Deuterium has one proton and one neutron. Tritium has one proton and two neutrons.
Isobars
Atoms with the same mass number but different atomic numbers are called Isobars. The difference between isotopes and isobars is only the difference in the number of neutrons they possess.
Checkout our other Playlists…..
Biology Playlist:
• Monera | Bacteria | Kingdom of Life |...
Physics Playlist:
• Physics - Nuclear Fission reaction ex...
Chemistry Playlist:
• Atomic number and Mass number of an a...
Human Anatomy Playlist: • Understanding Blood Pressure | Human ...
Science Experiments Playlist:
• Plasma ball light bulb experiment | F...