Bouncing egg experiment or naked egg science trick | Egg in Vinegar
Bouncing egg experiment or naked egg science trick | Egg in Vinegar
You will need
• A raw egg
• Vinegar
• A glass
Procedure
• Place the raw egg in the glass.
• Now pour some vinegar so that the egg is completely in it.
• Leave it for 2 days.
• Take out the egg from vinegar.
• Remove the shell.
• Bounce it on the table.
• We see that egg bounces on the table.
Explanation
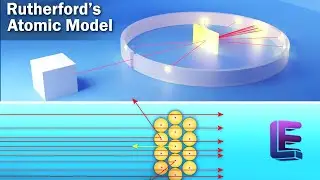
Egg shell contains calcium carbonate and Vinegar contains acetic acid. When calcium carbonate and acetic acid react, carbon dioxide is released. This process takes for 2 days as the complete carbon in the shell should be used. When you take the egg out of the vinegar it's soft because all of the carbon floated out of the egg and it bounces.
Chemical reaction taking place here is
2CH3COOH + CaCO3 gives rise to Ca(CH3COO)2 + H2O + CO2
Aceticacid Calcium Carbonate water carbon dioxide





![Gangsta's paradise - SX18 Eden: Bounty - Gate 6 lvl entrance - 670 UaO vs 579 goW [Rise of castles]](https://images.videosashka.com/watch/nBwtVbFucj8)