S02E01 - Thermodynamic Property Databases and Methods for Distillation with Rust
Overview: In the second season of Pragmatic Rust for Engineers, we focus on distillation columns, responsible for about 50% of the energy costs in the chemical industry in Europe. Join Tim Janus and Jonathan Ayala as we lay the foundation with an introduction to thermodynamic property methods to describe the behavior of non-ideal mixtures.
What You'll Learn:
Phases, partial pressure, activity coefficients, entropy, and enthalpy
Peng-Robinson and Non-Random Two-Liquid (NRTL) methods to approximate properties
Engage with Us: Based on your feedback from Season 1, we've moved the programming-related content to a live-stream format for better interaction. We’re excited to hear your thoughts on Rust programming and chemical process simulations. Share your experiences in the comments, and don’t hesitate to reach out for help or feedback!
General Learning Objectives:
Understand bioethanol production
Explore how phases of chemical mixtures depend on temperature and pressure
Learn about thermodynamic properties like activity coefficients, Gibbs free energy, enthalpy, entropy, and partial pressure
Discover the Peng-Robinson and NRTL methods and how to select the right thermodynamic property method
Janus IT and friends:
I’m Tim Janus, your host. Janus IT and friends include myself and colleagues who are eager to learn and share knowledge together. In this season, we will explore how to apply Rust for the simulation of distillation columns.
Social and Website Links:
Website: https://janus.rs/en/
Tims Twitter: / darthb86
Tims LinkedIn: / tim-janus-07523b13
Jonathans LinkedIn: / jonathan-ayala-b679426b
Follow us for more insights and updates on our journey on Chemical Engineering and Rust.
Chapters:
00:00 Intro - with content of the upcoming season
00:38 1st Example for Distillation - Making Brandy out of Wine
00:55 2nd Example for Distillation - Bio Ethanol
01:40 Boiling Points of Water and Ethanol
02:01 There is a catch: Azeotrope in Water Ethanol Mixtures
02:33 Thermodynamic Properties - Introduction with Phase Diagram
04:41 Thermodynamic Properties Examples - Enthalpy, Gibbs free Energy and Entropy
05:45 Entropy - General Definition - Application in Computer Science and Chemical Engineering
07:01 Thermodynamic Properties - Greater changes if phase change is involved
07:38 Thermodynamic Properties - Important for Distillation
08:10 Examples for Property Methods
10:18 Ideal and Non-Ideal Mixtures - Raoul's law
11:36 How to select from the Zoo of Property Methods?
12:45 Overview on Property Methods in this Season
13:53 NRTL for low pressure non-ideal mixtures
15:35 Peng Robinson with Mixing Rules for High Pressure
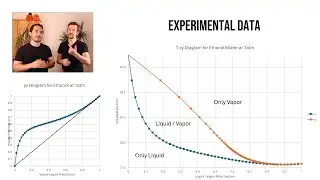
17:45 What is the experimental data, that Property Methods are based upon?
19:50 Parameter Estimation Binary Interaction Parameters of Property Methods
20:48 What to expert from the Livestreams: Postgres, Diesel, Clap aaaand a lot of Rust
21:49 Outtakes - We had fun and you will too!
22:56 Outro - Some organization stuff
Links:
Talk on Rust Nation UK: • Tim Janus - Let's get interdisciplina...
GitHub Repository: https://github.com/DarthB/PRfE_diesel...
References:
Amornraksa, Suksun & Subsaipin, Ittipat & Simasatitkul, Lida & Assabumrungrat, Suttichai. (2020). Systematic design of separation process for bioethanol production from corn stover. BMC Chemical Engineering. 2. 10.1186/s42480-020-00033-1.
Renon, H., and J. M. Prausnitz, “Local Compositions in Thermodynamic Excess Functions for Liquid Mixtures,” AIChE J., 14, 135 (1965).
Peng, D. Y., and D. B. Robinson, “A New Two-Constant Equation of State,” Ind. Eng. Chem. Fund., 15, 59 (1976).
Wong, D.S.H. and Sandler, S.I. (1992), A theoretically correct mixing rule for cubic equations of state. AIChE J., 38: 671-680. https://doi.org/10.1002/aic.690380505
Van der Waals, J.D., 1873, Doctoral dissertation, Leiden

![[FREE] SLIMESITO x BEEZYB TYPE BEAT 2022 -](https://images.videosashka.com/watch/1EoTITwenvE)