Enthalpy of Vaporitzation
View full question and answer details: https://www.wyzant.com/resources/answ...
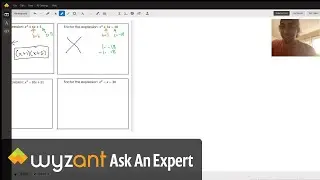
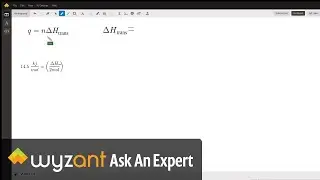
Question: Determine the enthalpy of vaporization, in kJ/mol, of C₆H₁₄ if 14.5 kJ of heat is needed to vaporize 0.500 moles of C₆H₁₄.
------------------------
Answered By:
Alex M.
Alex - Patient BS/MD Student working as a College Teaching Assistant
More information: https://www.wyzant.com/Tutors/MI/Troy...
------------------------
Written Explanation:
The answer to this question is 29 kj! This is explained in the video above.
See full answer: https://www.wyzant.com/resources/answ...
------------------------
About: Wyzant Ask an Expert offers free answers to your toughest academic and professional questions from over 65,000 verified experts. It’s trusted by millions of students each month with the majority of questions receiving an answer within 1 hour of being asked. If you ever need more than just an answer, Wyzant also offers personalized 1-on-1 sessions with experts that will work with you to help you understand whatever you’re trying to learn.
Ask your own question for free: https://www.wyzant.com/resources/answ...
Find a tutor for a 1-on-1 session: https://www.wyzant.com?utm_source=youtube&utm_medium=organic&utm_campaign=aae_video
Subscribe to Wyzant on YouTube: https://www.youtube.com/subscription_...