Laws of the Lungs and Respiratory System *EXPLAINED* 🫁
How do the lungs work? Watch this quick video for an overview of the essential laws of the lungs and respiratory system.
Respiratory System Laws [Full Guide]
Respiratory Therapy Definitions [Glossary]
️ Laws of the Lungs and Respiratory System
The laws governing the function of the lungs and the respiratory system are rooted in principles of physiology and physics, providing a fascinating insight into how we breathe. The first two principles that I should mention are Boyle's Law and Henry's Law, which explain the mechanics of breathing and gas exchange in the lungs.
️ Boyle's Law
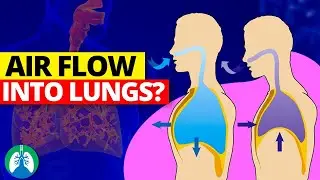
Boyle's Law relates to the mechanical aspect of breathing. It states that the pressure of a gas is inversely proportional to its volume, within a closed system. In the context of the respiratory system, this law is exemplified during the process of inhalation and exhalation. When the diaphragm and intercostal muscles contract, they increase the thoracic cavity's volume. This expansion decreases the pressure inside the lungs relative to the atmospheric pressure, leading to the influx of air (inhalation).
Conversely, when these muscles relax, the thoracic cavity's volume decreases, increasing the pressure in the lungs and causing air to be expelled (exhalation). This process is essential for ventilating the lungs and is the basis for how we breathe.
️ Henry's Law
Henry's Law is crucial in understanding the gas exchange that occurs in the lungs. It states that the amount of gas that dissolves in a liquid (like blood) is directly proportional to the partial pressure of that gas in the surrounding atmosphere, provided the temperature is constant. In the lungs, oxygen from inhaled air diffuses into the blood because the partial pressure of oxygen is higher in the alveoli than in the blood.
Conversely, carbon dioxide, which is present in higher concentrations in the blood, diffuses from the blood into the alveoli to be exhaled. This efficient gas exchange is vital for maintaining the body's pH balance and for oxygenating the blood. The concept of partial pressure is further elaborated by Dalton's Law, which states that in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of individual gases. This principle is important in calculating the concentrations of oxygen and carbon dioxide in the blood and is used in clinical settings to assess respiratory efficiency.
️ Fick's Law of Diffusion
Fick's Law of Diffusion also plays a significant role in respiratory function. It explains how the rate of gas transfer across a membrane (like the alveolar wall) is proportional to the surface area of the membrane, the difference in partial pressures of the gas across the membrane, and inversely proportional to the thickness of the membrane. This law underscores the importance of a large surface area and thin walls in the alveoli for efficient gas exchange.
Respiratory System Laws [Full Guide]
—————
FREE STUFF
▪ Free Cheat Sheets
▪ TMC Practice Exam
PASS THE TMC EXAM
▪ TMC Test Bank
▪ TMC Exam Hacks
▪ Daily TMC Practice Questions
▪ TMC Bundle (Save $)
MORE FROM RTZ
▪ Test Bank (Free)
▪ Glossary
▪ About Us
▪ Testimonials
FOLLOW US
▪ Instagram
▪ Twitter
▪ Facebook
▪ Pinterest
▪ Rumble
▪ LinkedIn
MEDICAL DISCLAIMER
This content is for educational and informational purposes only. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Please consult with a physician with any questions that you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you watch in this video. We strive for 100% accuracy, but errors may occur, and medications, protocols, and treatment methods may change over time.
AFFILIATE DISCLAIMER
This description contains affiliate links. If you decide to purchase a product through one of them, we receive a small commission at no cost to you.
—————
⏰TIMESTAMPS
0:00 - Intro
0:17 - Principles
0:24 - Boyle's Law
1:06 - Henry's Law
1:42 - Partial Pressure
2:03 - Fick's Law
2:26 - Laplace Law
2:46 - Laws of the Lungs
—————
CREDIT FOR MUSIC/GRAPHICS:
▪ Music licensed from Audiojungle.net/
▪ Graphics: Canva.com, Freevector.com, Vecteezy.com, and Pngtree.com