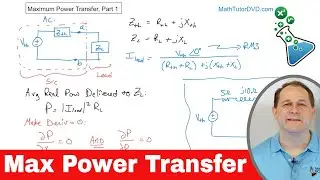
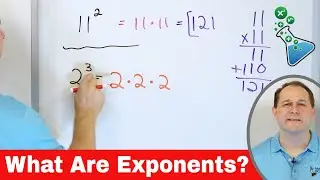
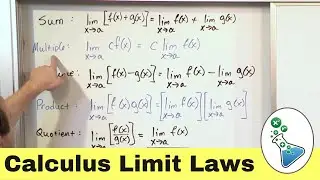
Electrons & Protons in Chemistry
Think of atoms as the building blocks of everything around you, like tiny Lego pieces. At the heart of every atom is the nucleus, where protons hang out. Protons are positively charged particles that give the nucleus its positive charge. The number of protons in an atom determines what element it is—hydrogen has one proton, helium has two, and so on. Protons are like the ID cards of elements.
Now, swirling around the nucleus like energetic bees are electrons. Electrons are negatively charged and much smaller than protons. They zoom around in different energy levels or shells, creating a sort of electron cloud. The number of electrons usually equals the number of protons, balancing out the charge and making the atom neutral.
But here’s where it gets interesting: electrons can jump between energy levels, especially when atoms interact with each other. This movement is crucial for chemical reactions. When atoms gain or lose electrons, they become ions. Lose an electron, and you get a positively charged ion (cation).
Gain an electron, and you get a negatively charged ion (anion). These ions then attract each other, forming ionic bonds that create new compounds.
In chemistry, electrons are the action heroes, making and breaking bonds, while protons keep things stable and identify what kind of atom you're dealing with. Together, they form the dynamic duo that drives the fascinating world of chemistry!
More Lessons: http://www.MathAndScience.com
Twitter: / jasongibsonmath